CU Medical Systems
iPAD SP2 Automatic External Defibrillator - Packages
- SKU:
- 63500
- UPC:
- 660042497246
- MPN:
- iPAD2AED
- Weight:
- 2.40 KGS
Description
The iPAD SP2 with ECG and Optional Manual Overide and Cardio Version
A new class of defibrillator.
A lightweight compact defibrillator that delivers greater functionality than just an AED when it is needed most.
- Highly portable and durable - weighing only 2.4kg.
- Full colour screen with 3 modes - AED, monitor and manual.
- Manual override from 2J to 200J
- Bluetooth III lead ECG monitoring
- II lead ECG monitoring via electrode pads
- Bluetooth printer for full or partial on-scene printout
- Multiple event recording & simple data download
- On screen printable data review & ECG playback
- Synchronised cardioversion
- Plug-in pad adaptors to stop pad changes when backup arrives
- Dual adult/child smart electrode pads
- Modular system customised to end users clinical skills.
Compact. Robust. Lightweight.
The iPAD SP2 is designed for durability. It is drop tested from 1.2m upon any surface, on all corners. It is also water and dust proof (IP55).
It can operate between 0°C —50°C and meets all standard vibration tests (MILSTD 810G).
Disposable Adult/Child Smart Electrode Pads
Adult/Child "Smart" Pads, capable of 'talking' to the device to notify users of expired defibrillator pads and if the pads have already been used.
These pads are useable with paediatric patients by simply selecting adult or child soft key. In AED mode, these pads can be used on infants down to 1 years old.
Bluetooth Technology
The iPAD SP2 is designed to be future proof, providing industry standard technologies for high performance during rescue situations. The Bluetooth printer and ECG module allow for remote monitoring (up to 10 metres) and on the spot reporting— by the casualty’s side or on the move.
Manual Override
Gives trained healthcare professionals the opportunity to interpret select the appropriate energy level and delivery a shock manually. The low energies available under manual mode allow for infants under the age of 1 to be shocked.
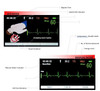
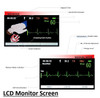
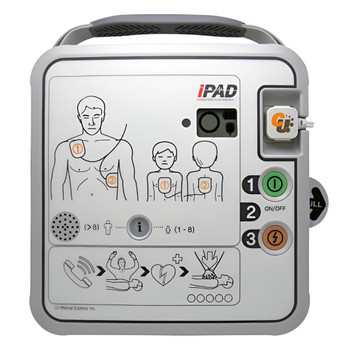
Full colour screen
The colour screen allows for significant amounts of clinical data to be available at your fingertips. The indicators (see LCD Monitor section) provide up to date information on the patient through either lead II ( via defibrillation pads) or via the 3 Lead ECG module. It will also clearly show modes (AED/ Manual/Monitoring) and patient modes (Adult/Paediatric).
Event review and playback
The ‘review’ function allows for recorded events to be replayed on the colour screen with the option to print out a partial or full readout from the Bluetooth printer.
Ambient Noise Detector
When the environment is loud, the iPAD SP2 automatically senses this and increases it’s own volume to ensure the rescuer can hear audible commands clearly and precisely. If preferred, the volume can be manually controlled.
Multifunctioning soft keys
User friendly interface to be able to set up and change modes and settings quickly. They are inset from the body of the unit to prevent accidental pressing or knocking.
Synchronized cardioversion
A feature normally only available on high end equipment.
Bluetooth Technology – CU-EM1 – ECG Transmission Device
This powerful device allows for three lead ECG monitoring and can be used simultaneously with defibrillator electrode pads. The transmission device also has a belt clip attached upon the back for hands free transportation.
The device has a range of 10m between the transmission device (labelled ) and the SP2.
Power Options:
The SP2 provides both disposable and rechargeable battery options, with the disposable battery pack similar to the SP1, as well as a new rechargeable battery pack, designed for intensive usage of your machine. Along with the SP2, all the additional accessories; the Bluetooth printer and ECG transmission device both operate on rechargeable batteries.
The rechargeable battery pack is the recommended power option for when monitoring is required.
The SP2 Charging Dock provides a means to restore power to all your devices —in one sitting!
Bluetooth ECG Printer:
This rechargeable printer offers a method of immediately providing a permanent record of the event. With a meter to allow you to check the current charge left in the device, a manual feed option as well as an error status indicator which will allow you to know if there’s any fault with the printer.
The paper used on this device is also compatible with the other CU medical products (HD1 and ER Ranges).
The device has a range of 10m between itself and the SP2.
Optional Extras:
- Battery charging docking station
- SP2 Rechargeable Battery Pack (2 year life span)
- Manual override (from 2J to 200J) - Bluetooth ECG Module - The 3-Lead ECH monitoring via bluetooth technology means patients can be continuously monitored, even in difficult environment or situations. It also means patients can be moved and monitored at all times.
- Bluetooth Printer – This rechargeable printer using Bluetooth technology means that a full or partial print out can be obtained , on the scene during any scenario. Recharged via a mains adaptar.
- Electrode pad adaptors - These pad adapters will allow the electrode pads from Zoll, Phillips and PhysioControl to be used with the iPAD SP1.
iPAD 2 Defibrillator Package Options:
1. iPAD SP2 AED (Defibrillator) Standard-Non-Recharge Package - 63500
- iPAD SP2 Defibrillator Package, containing;
- iPAD SP2 AED without Manual Override
- Carry Case
- Dual Adult/Child Electrodes - 1 sets
- Disposable Battery Pack.
2. iPAD SP2 AED (Defibrillator) Basic Manual Package - 63502
- iPAD SP2 Defibrillator Package, containing;
- iPAD SP2 AED with Manual Override
- Carry Case
- Dual Adult/Child Electrodes - 1 sets
- Disposable Battery Pack.
3. iPAD SP2 AED (Defibrillator) Ultimate Package (+Manual) - 63508
- iPAD SP2 Defibrillator Package, containing;
- iPAD SP2 AED with Manual Override
- Carry Case
- Dual Adult/Child Electrodes x 1 pair
- Rechargeable Battery Pack x 2
- Recharging Dock
- Bluetooth 3 Lead ECG Module
- Bluetooth Printer.
4. iPAD SP2 AED (Defibrillator) Ultimate Package (-Manual) - 63510
- iPAD SP2 Defibrillator Package, containing;
- iPAD SP2 AED with No Manual Override
- Carry Case
- Dual Adult/Child Electrodes x 1 pair
- Rechargeable Battery Pack x 2
- Recharging Dock
- Bluetooth 3 Lead ECG Module
- Bluetooth Printer.
Specification:
Dimensions 260㎜ x 256㎜ x 69.5㎜ (Width x Length x Height)
Weight 2.4㎏ (Including the battery pack and pads)
Environmental Conditions:
Operating Environment (The device can be used immediately in case of an emergency.)
Temperature: 0°C ~ 43°C (32°F ~ 109°F)
Humidity: 5% ~ 95% (a location with no condensation)
Storage Environment (The device has pads and a battery and is ready to be used for an emergency.)
Temperature: 0°C ~ 43°C (32°F ~ 109°F)
Humidity: 5% ~ 95% (a location with no condensation)
Transportation Environment (The device does not have pads and a battery and is separately stored or transported over a long period of time.) Temperature: -20°C ~ 60°C (-4°F ~ 140°F)
Humidity: 5% ~ 95% (a location with no condensation)
Altitude 0 to 15,000 feet (operational and storage)
Drop Withstands 1.2-meter drop to any edge, corner, or surface
Vibration Operating: Meets MIL-STD-810G
Sealing IEC 60529: IP55
ESD Meets IEC 61000-4-2:2001
EMI (Radiated) Meets IEC 60601-1-2 limits, method EN 55011:2007 +A2:2007,
Group 1, Class B
EMI (Immunity) Meets IEC 60601-1-2 limits, method EN 61000-4-3:2006 +A1:2008
Level 3 (10V/m 80MHz to 2500MHz)
Defibrillator:
Operation Type Semi-automated External Defibrillator
Output Type e-cube biphasic (Truncated exponential type)
Output Energy - AED Mode
150J, 200J at 50Ω load for adults
50J at 50Ω load for children
- Manual Override (Not Option)
2J, 3J, 5J, 7J, 10J, 20J 30J 50J 70J, 100J, 150J, 200J
Charge Control Controlled by an automated patient analysis system
Charge Time For the first defibrillation of a new battery, capable of administering shock within 9 seconds of the given voice instruction.
Disposable Battery - 150 Shocks / 5 hours of operation time.
Rechargeable Battery - 70 Shocks / 3 hours of operation time.
Further information about Defibrillators
What is a defibrillator? It can be the difference in saving a life
How does a defibrillator work?
Top Tips for Buying a Defibrillator
Advice for purchasing defibrillators for schools
Defibrillator FAQs
All the defibrillators and AED's we suply are easy to use, self test and whilst users can be safe in the knowledge they cannot get shocked in error by touching the patient during defibrillation.