CU Medical Systems
iPAD CU-SPR Semi-Automatic Defibrillator
- SKU:
- SPR
- UPC:
- 7421129516297
- MPN:
- SPR
- Weight:
- 2.00 KGS
- Width:
- 24.00 (cm)
- Height:
- 7.00 (cm)
- Depth:
- 23.00 (cm)
Description
Introducing the Next Generation iPAD AED - the iPAD SPR!
We are proud to launch the iPAD SPR, a new and improved model that builds upon the success of the SP1. With its class-leading IP66 rating, this AED is highly resistant to water and rugged environments. It also features a USB data transfer function, allowing real-time event and ECG Trace logs. Plus, it can record up to 5 events lasting up to 3 hours each.
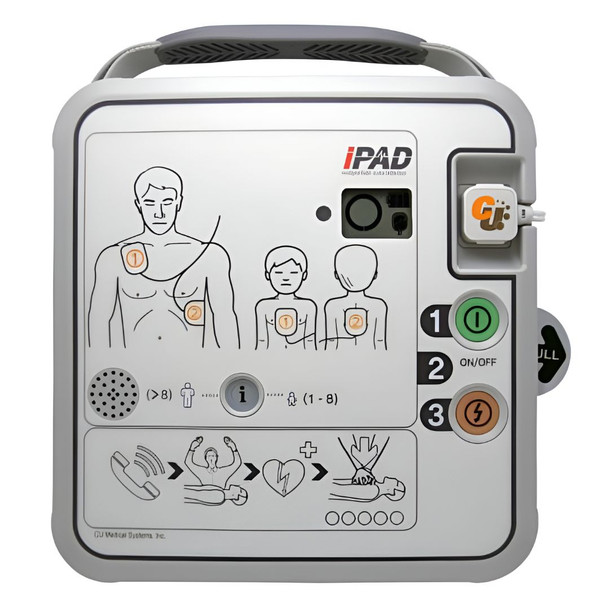
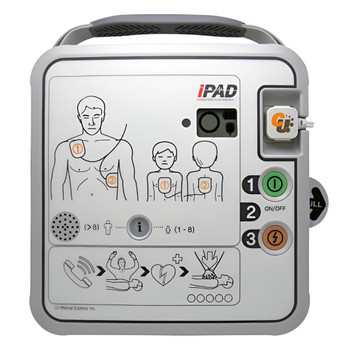
Designed to be the gold standard in advanced and intuitive public access AEDs, the iPAD SPR is a lifesaver. Its LCD screen makes it easy to monitor the device status, ensuring it's always ready to use. It supports both adult and pediatric patients, with a quick and simple mode change at the press of a button.
The CU-SPR provides voice prompts and CPR guidance, ensuring effective usage by anyone. And with its IP68 water and dust resistance, it's suitable for use in various environments.
Key Features for Your Convenience:
- LCD screen displays device and consumables status for quick monitoring
- CPR metronome, voice guidance, and graphic instructions
- Quick change button for switching between adult and pediatric modes
- USB data transfer for easy information retrieval
Safety Features You Can Rely On:
- Automatic internal discharge
- Daily/weekly/monthly self-test for peace of mind
- Shock resistant carrying case
- Highly water resistant casing with IP66/IP68 rating (optional)
Experience Advanced Technology:
- Semi-automated e-cube biphasic defibrillation
- Combined adult/pediatric pads
- Automatic background noise analysis and device volume adjustment
- CPR step detection indicator for more effective CPR
Package Contents:
- Protective carry case
- 1 x Disposable lithium manganese oxide battery with a warranty of four years and an expected life of up to five years in standby mode
- 1 x set of electrode pads (one pre-connected into the AED) suitable for adults and children aged 1+
- 1 x AED rescue kit, including 2 x patient wipes, nitrile gloves, prep razor, face shield, and tough cut scissors
Specification:
- Dimensions: 240mmx230mmx70mm (W x L x H)
- Weight: 2kg (including battery pack and pads)
Environmental:
- Operating Conditions - Temperature: 0˚C ~ 50˚C (32˚F ~ 122˚F) Humidity: 5% ~ 95% (non condensing)
- Storage Conditions - Temperature: 0˚C ~ 50˚C (32˚F ~ 122˚F) Humidity: 5% ~ 95% (non condensing)
- Transport Conditions - Temperature: -20˚C ~ 60˚C (-4˚F ~ 140˚F) Humidity: 5% ~ 95% (non condensing)
- Altitude - 0 to 4,572 m (0 to 15,000 ft.) – operational and storage
- Drop - Withstands 0.75 meter drop to any edge, corner, or surface
- Vibration - Operating: Meets MIL-STD-810G Fig.514.6E-1 Standby: Meets MIL-STD-810G Fig.514.6E-2
- Sealing (Regular) - IEC 60529:2013 IP66
- Sealing (Optional) - IEC 60529:2013 IP68
- ESD - Meets IEC 61000-4-2:2008
- EMI (Radiated) - Meets IEC 60601-1-2 limits, method EN 55011:2016+A1:2017, Group 1, Class B
- EMI (Immunity) - Meets IEC 60601-1-2 limits, method EN 61000-4-3:2006 +A2:2010 Level 3 (10V/m 80MHz to 2.5GHz)
Defibrillator:
- Operating Mode - Semi-automated
- Waveform - E-cube biphasic (Truncated exponential type)
- Output Energy - 150J at 50 Ω load for adults
- 50J at 50 Ω load for children
- Charge Control - Controlled by an automated patient analysis system
- Charging Time - Within 3 seconds from when the voice instruction, "An electric shock is needed" is issued.
- Time from Initiation of Rhythm Analysis - 10 seconds with a new battery (even after the delivery of 15 discharges at 150J)
- 12 seconds with a new battery (even after the delivery of 15 discharges at 200J)
- Time from CPR to Shock - At least 6 seconds from the completion of CPR to shock delivery
- Disarm - Patient’s heart rhythm changes to non-shockable rhythm. The SHOCK button is not pressed within 15 seconds
ECG Acquisition:
- Acquired ECG Lead - Lead II
- Frequency Response - 1 Hz to 30 Hz
ECG Analysis System:
- Impedance Range - 25Ω to 175Ω
- Shockable Rhythms - Ventricular Fibrillation or Fast Ventricular Tachycardia
- Sensitivity and Specificity - Meets ANSI/AAMI DF80 guidelines
Control, Indicators and Prompts:
- Controls - Power Button Shock Button
- Adult/Paediatric Selection - Button
- Status LCD - Displays device status, battery level and pads status
- Status LED - Displays device status, battery level and pads status
- Indicators - Do-Not-Touch-Patient. Pads Patch Position Indicators. Pads Connector Status Indicator. Status LED Indicator. CPR Detection Indicator. Shock Button. Blue i-Button.
- Speaker - Provides voice prompts
- Sound Level - 80dB ~ 90dB (±3dB), apart 1m above speaker
- Beeper - Provides various audible indications
- Battery Level - Shown on the Status LCD
- Low Battery Indicator - Self-Test Flashing red i-Button
Self-Test
- Automatic - Power On Self-Test, Run-time Self-Test Daily, Weekly, and Monthly Self-Test
- Manual - Battery Pack Insertion Test
Battery Pack:
- Battery Type - 12V DC, 4.2Ah LiMnO2, Disposable
- Capacity - At least 200 shocks (150J) or 8 hours of operating time
- Standby Life - At least 5 years from the date of manufacture
- Temperature Ranges - Operating Temperature: 0˚C ~ 50˚C (32˚F ~ 122˚F)
- Storage Temperature: -20˚C ~ 60˚C (-4˚F ~ 140˚F)
Adult / Paediatric Defibrillation Pads
- Surface Area - 85cm²
- Cable Length - 120cm
- Shelf life At least 36 months from the date of manufacture Data
Storage and Transfer:
- USB - External memory. Data may be copied from the internal memory to the USB.
- File System - FAT32
- Internal Memory Data - 5 individual treatments, up to 3 hours per treatment
- Capacity
Warranty:
- 10 Years Warranty once registered.